






![]()
ADVANCED
T he way that the electrons in semiconductors move is coordinated with the motion of those of an adjacent atom, so that a covalent bond is imposed between them. Semiconductor elements like Silicon (Si) and Germanium (Ge) have 4 electrons in the outermost orbit and each atom is linked to 4 other atoms by sharing one electron with each atom as shown in the crystal below.
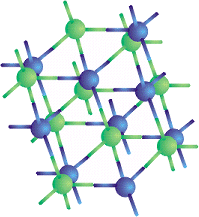
|
This is schematically
represented as:
|
The semiconductors that carry electric current can be classified as:
1)Intrinsic Semiconductors
These are pure semiconductor elements belonging to IV group of periodic table (eg. Germanium, Silicon). At room temperature, some of the electrons absorb thermal energy from the surroundings and get liberated. This also leads to the formation of electron-deficient positively charged spaces called "holes". The movement of the holes and of electrons in the opposite direction constitute the electric current when a P.D. is applied. However, this electric current is extremely feeble. Hence an intrinsic semiconductor lacks a significant source of electrons and can be of only limited use as an electronic device.
2) Extrinsic Semiconductors
Some impurities are added to the pure semiconductors or in scientific terms, the semiconductor crystal is 'doped' with impurities to increase the charge-carrying capacity of the semiconductor. The two types of impurities are:
1) Donor or n-type impurity
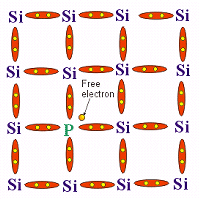 This is generally an element belonging to the fifth group of periodic table
and hence containing 5 valence electrons. These include Arsenic(As), Phosphorus (P),
Antimony (Sb). The impurity atom then forms 4 covalent bonds with the neighbouring atoms
in the semiconductor crystal. Consequently, the fifth electron is set free and it
possesses enough thermal energy not to be attracted to the nucleus of impurity atom. This
results in an increase in density of electrons which act as majority charge carriers,
constituting an electric current.
This is generally an element belonging to the fifth group of periodic table
and hence containing 5 valence electrons. These include Arsenic(As), Phosphorus (P),
Antimony (Sb). The impurity atom then forms 4 covalent bonds with the neighbouring atoms
in the semiconductor crystal. Consequently, the fifth electron is set free and it
possesses enough thermal energy not to be attracted to the nucleus of impurity atom. This
results in an increase in density of electrons which act as majority charge carriers,
constituting an electric current.
2) Acceptor or p-type impurity
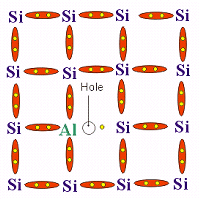 This is generally an element belonging to the third group of periodic table
and hence containing 3 valence electrons. These include Aluminium (Al), Boron (B), Indium
(In). The impurity atom then forms 3 complete covalent bonds and a fourth incomplete
covalent bond with the neighbouring atoms in the semiconductor crystal. Hence due to the
lack of electron in the fourth covalent bond, an electron deficient region or 'hole' is
created. The movement of these holes constitutes the electric current. Hence the 'holes'
act as positive charge carriers. Note that even in extrinsic semiconductors, a very small
number of holes and free electrons are formed due to absorption of thermal energy from
surroundings. These are called as minority charge carriers.
This is generally an element belonging to the third group of periodic table
and hence containing 3 valence electrons. These include Aluminium (Al), Boron (B), Indium
(In). The impurity atom then forms 3 complete covalent bonds and a fourth incomplete
covalent bond with the neighbouring atoms in the semiconductor crystal. Hence due to the
lack of electron in the fourth covalent bond, an electron deficient region or 'hole' is
created. The movement of these holes constitutes the electric current. Hence the 'holes'
act as positive charge carriers. Note that even in extrinsic semiconductors, a very small
number of holes and free electrons are formed due to absorption of thermal energy from
surroundings. These are called as minority charge carriers.